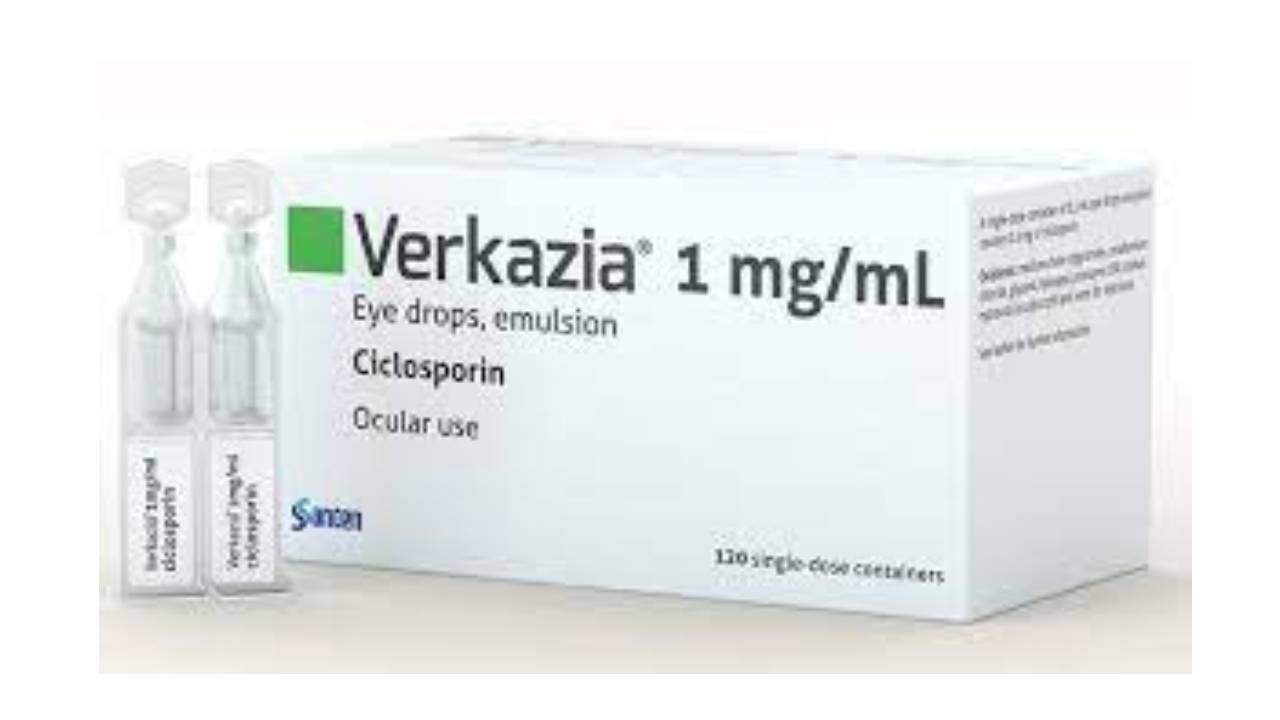
Verkazia: FDA Approval
With the FDA’s approval of cyclosporine ophthalmic emulsion 0.1% (Verkazia), eyecare practitioners now have an extra alternative to affect their sufferers, each adult, and kids, with vernal keratoconjunctivitis (VKC), an allergic eye circumstance that if left untreated can cause corneal guard ulcers and doubtlessly vision loss.
The remedy, which could be attainable utilizing a prescription drug that comes in an oil-in-water cationic emulsion. It offers enhanced ocular bioavailability of cyclosporine, supported by Santen Inc, the corporate.
The therapy inhibits T-cell activation and reduces the extent of immune cells and mediators that cause allergic irritation of the ocular floor.
Case study
The treatment was tested in two randomized, multicenter, double-masked, vehicle-controlled scientific trials: the VEKTIS examine and therefore the NOVATIVE checkout.
An announcement issued by using the corporate described the examine particulars: within the VEKTIS analysis, patients with extreme VKC had been randomly assigned to receive Verkazia 1 mg/mL 4 times a day or two instances a day and automobile neighborhood for the first four months.
The NOVATIVE analysis concerned patients with reasonable to extreme VKC who had been randomly assigned to accumulate Verkazia 1 mg/mL 4 instances every day or cyclosporine ophthalmic emulsion 0.5 mg/mL fourfold daily and car neighborhood for the first month.
Patients who had been assigned to the car group were switched to Verkazia (four times daily or twice daily) from month four to month 12 within the VEKTIS analysis and to cyclosporine ophthalmic emulsion 0.5 mg/mL 4 times day by day or 1 mg/mL from month 1 to month 4 within the NOVATIVE examine
Verkazia produced advancements in irritation of the cornea in both reports, as measured by using keratitis rating, and in ocular itching. Opposed reactions that had been suggested in >5% of patients are eye pain (12%) and eye pruritus (8%), which were always transient.
Verkazia became available for prescription use in Canada in 2020. Rocha has been prescribing it for the previous year and said that it’s made an outsized difference for his patients who’ve VKC. “they are tolerating it smartly,” he noted. “they have gotten a remedy they might use in the future safely and readily without systemic or local side effects.”
Topical steroids are beneficial within the medication of VKC, but Rocha acknowledged that their extended use incorporates many hazards. “You cannot use them in the future,” he said. “They produce a kind of localized immune suppression which will end in infections or cataracts or excessive pressure .”
Rocha mentioned that he and other clinicians used compound 1% cyclosporine to treat VKC, but the components were far from the premiere with an appreciation for bioavailability and tolerability. “It isn’t completely made for attention,” said Rocha.
“Now we now have 0.1% emulsion made especially for attention. It’s preservative-free, which is what you would like for a condition that comprises hypersensitivity. It doesn’t trigger any reactions. it’s one-tenth of what we had been using before, as we used a tenth solution.”
The therapy will halt the event of VKC to other ocular pathologies, like corneal preserve ulcers, in line with Rocha.
“Every time we’ll prevent a significant disorder on the cornea which will cause scarring or corneal opacification, this is often a reasonably good element,” he said.
The indicators of VKC are sometimes brought on or exacerbated using seasonal allergies everywhere in the summer and fall, cited Rocha. Patients can start the remedy once they have gotten flares and stop therapy when the ailment isn’t lively, he delivered.
“If sufferers are in a lively allergenic environment, they could even have an exacerbation,” said Rocha. “When winter arrives, then they’ll feel enhanced. they will begin therapy, cease it for a few of months, after which return thereon if obligatory.”
There isn’t any chance of response diminishing after a drug holiday from Verkazia, explained Rocha.
About Vernal Keratoconjunctivitis (VKC)
VKC is an infrequent and recurrent allergic eye situation, commonest in infants and youth, that explains severe inflammation of the ground of the attention.
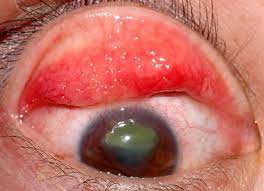
the most symptoms of VKC – light sensitivity, tearing, itching, and mucous discharge – can prevent these affected from participating in conventional activities. Approximately one-third of VKC instances are regarded as severe, and without ample medicine may find yourself in corneal ulcers and even imaginative and prescient loss.
About Verkazia
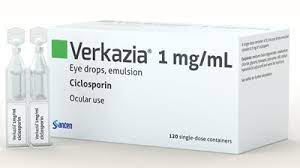
Verkazia cyclosporine 0.1% topical ophthalmic emulsion is indicated for the medication of severe vernal keratoconjunctivitis in children from four years aged via youth.
The suggested medication routine authorized with the help of health Canada is one drop of Verkazia four instances each day (morning, midday, afternoon, and night) to be applied to each affected eye.
The medication should still be discontinued if no improvement in indications and symptoms of vernal keratoconjunctivitis is accompanied when used for treatment of vernal keratoconjunctivitis.
The drugs can also be maintained on the suggested dose or reduced to 1 drop twice daily as soon as sufficient signs and indicators are accomplished.
Medication should still be discontinued after signs and symptoms are resolved and reinitiated upon their recurrence.